

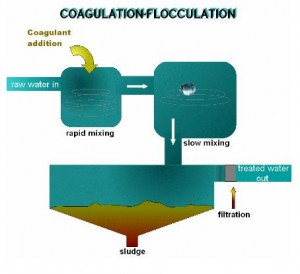
Water Treatment Coagulation
Water Treatment Coagulants
Coagulant water treatment chemicals come in two main types –
primary water treatment coagulants and coagulant aids.
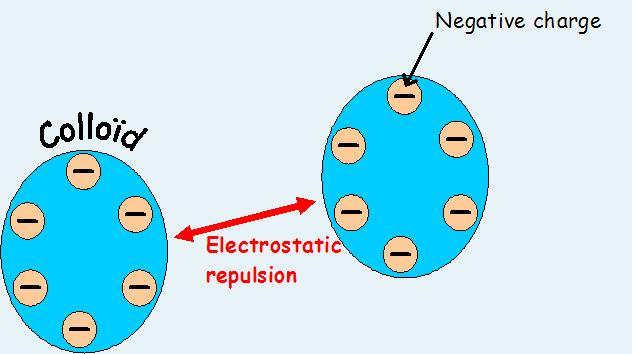
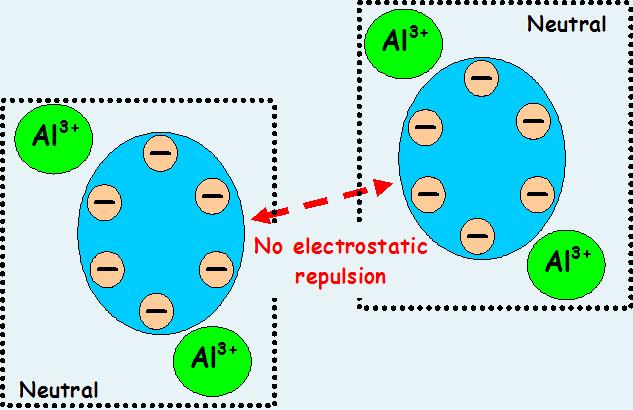
Primary coagulants neutralize the electrical charges of particles in the water which causes the particles to clump together. Chemically, coagulant water treatment chemicals are either metallic salts (such as alum) or polymers. Polymers are man-made organic compounds made up of a long chain of smaller molecules. Polymers can be either cationic (positively charged), anionic (negatively charged), or non ionic (neutrally charged.)
The common water treatment coagulant chemicals used are
- Aluminum Sulphate (ALUM)
- Ferrous Sulfate (copperas)
- Ferric Sulfate
- Ferric Chloride
The choice of the coagulant to be used for any particular water should preferably be based upon experiment on different coagulants.
Alum
One of the earliest, and still the most extensively used coagulant, is aluminum sulfate (Al/S04)3 ·14 HP), also known as alum. Alum is acidic with light tan to grey in color and available in blocks, lumps and powder with a density of 1000 -1100 kg/ m3 and specific gravity of 1.25 to 1.36. Alum can be bought in liquid form or in dry form. It is readily soluble in water. When alum is added to water, it reacts with the water and results in positively charged ions. The ions can have charges as high as +4, but are typically bivalent (with a charge of +2.) The bivalent ion . resulting from alum makes this a very effective primary coagulant.
Advantages and disadvantages of Alum
Advantages of alum are
- It readily dissolves with water, and
- It does not cause the unsightly reddish brown staining of floors, walls and equipment like ferric sulphate,
Disadvantages of alum are
- It is effective only at certain pH range, and
- good flocculation may not be possible with alum in some waters.
Ferrous Sulphate or Copperas
Ferrous sulphate, ordinarily known as copperas, is granular acid compound and green to brownish yellow color available in granules, crystals and lumps. This is fed usually in solution form with strength of 4 to 8 %. The alkalinity and pH value of natural water are too low to react with copperas to form the desired ferric hydroxide floc, because the reaction involves oxidation by the dissolved oxygen in the water, which does not occur when pH value is less than 8.5. It is necessary, therefore, to add lime with copperas to secure coagulation. For this reason, copperas is not used in coagulation of high coloured water, which coagulates best at pH values less than 6.0. The dose of lime required is approximately 0.27 mg/L to react with 1.0 mg/L of copperas. Generally the floc formed by the reaction of copperas and lime is feathery and fragile, but has a high specific gravity.
Ferric sulphate
Ferric sulphate is available as a commercial water treatment coagulant in the form of an anhydrous material that may be transported and stored in wooden barrels. The material will dissolve readily in a limited quantity of warm water so a special solution pot must be used with chemical feeders, in which 1 part ferric sulphate by volume is dissolved in 2 parts water to produce a solution of about 40% strength.
Advantages of Ferrous sulphate
- Ferric hydroxide is formed at low pH values, so that coagulation is possible with ferric sulphate at pH values as low as 4.0.
- Ferric hydroxide is insoluble over a wide range of pH values than aluminium hydroxide except for the zone of 7.0 to 8.5.
- The floc formed with ferric coagulants is heavier than alum floc.
- The ferric hydroxide floc does not redissolve at high pH values.
- Ferric coagulants may be used in color removal at the high pH values required for the removal of iron and manganese and in softening of water.
Reaction between alum and natural constituents of various waters are influenced by many factors, so it is impossible to determine accurately the amount of alum that will react with a given amount of alkalinity. Theoretically 1 mg/L of alum reacts with 0.45 mg/L of natural alkalinity expressed as CaCo3 0.30 mg/L of 85% quicklime as CaO , and 0.35 mg/L of 95% hydrated lime as Ca(OH)3′ Alum is generally fed in solution form with 8 to 10% strength.
If no alkali is added then the acidity of 1.0 mg/L alum will lower the natural alkalinity of the raw water by 0.45 mg/L. This lowering of natural alkalinity is desirable in most cases as the pH range for coagulation of turbid waters being 5.7 – 8.0. The alkali required for corrosion prevention, therefore, would be added to the filtered water, the required dose being influenced but not governed by the alum dose.
1 mg alum will produce approximately 0.26 mg of insoluble Al(OH)3 precipitates and will consume approximately 0.51 mg of alkalinity (expressed as Ca C03)·
1 mg of ferrous sulphate will produce approximately 0.64 mg of insoluble Fe(HC03)2 precipitates and will consume 0.56 mg of alkalinity .
1 mg of ferric sulphate will produce approximately 0.54 mg of insoluble Fe(OH\ precipitates and will consume 0.75 mg of alkalinity.
Because of the consumption of alkalinity, CO2 is produced during coagulation. The pH value may also be lowered after the coagulation process, depending on the amount of coagulant applied and the total alkalinity in the raw water.
Coagulant Aids
Coagulant aid is an inorganic material, when used along with main coagulant, improves or accelerates the process of coagulation and flocculation by producing quick forming, dense and rapid-settling flocs. Coagulant aids when added increase the density to slow-settling flocs and toughness to the flocs so that they will not break up during the mixing and settling processes. Primary coagulants are always used in the coagulation/ flocculation process. Coagulant aids, are generally used to reduce flocculation time and when the raw water turbidity is very low. The particles of coagulant aids may become negatively charged making them subject to attraction by positively charged aluminium ions. It is especially useful for clear water with very low turbidity that does not coagulate well with usual processes. Nearly all coagulant aids are very expensive, so care must be taken to use the proper amount of these chemicals. In many cases, coagulant aids are not required during the normal operation of the water treatment plant, but are used during emergency water treatment of water which has not been adequately treated in the flocculation and sedimentation basin. Common coagulant aids are
- Bentonite
- Calcium carbonate
- Sodium silicate
- Anionic polymer
- Non ionic polymer
Lime is a coagulant aid used to increase the alkalinity of the water. The increase in alkalinity results in an increase in ions (electrically charged particles) in the water, some of which are positively charged. These positively charged particles attract the colloidal particles in the water, forming floc.
Bentonite is a type of clay used as a weighting agent in water high in color and low in turbidity and mineral content. The bentonite joins with the small floc, making the floc heavier and thus making it settle more quickly.
Poly electrolytes, which are polymers containing ionisable units have been used successfully as both as coagulant aids and coagulants but care should be taken to guard against their toxicity.
Poly electrolyte create extraordinary slippery surfaces when spilled on floor and are difficult to clean up.
FACTORS INFLUENCING COAGULATION
Coagulation will be affected by changes in the water’s pH, salt content, alkalinity, turbidity, and temperature. Within the plant, mixing effects and coagulant effects will influence the coagulation/ flocculation process. The levels of pH, salts, and alkalinity in water are all ways of measuring the amounts of positively charged particles (cations) and negatively charged particles (anions) in the water. As!’l result, all three factors influence the amount of coagulants which must be used to remove the turbidity in the water.
- pH
The pH range of the water may be the single most important factor in proper coagulation. The optimum pH range varies depending on the coagulants used, but is usually between 5 and 7. These lower pH values mean that there are more positively charged particles loose in the water to react with the negatively charged colloids.
Coagulation should be carried out within this optimum zone using alkalis and acids for correction of pH where necessary. For many waters, which are low in colors and well buffered and having pH in the optimum zone, no adjustment of pH is necessary when alum is used as coagulant. Failure to operate within the optimum pH zone, may be a waste of coagulants and may be reflected in the lowered quality of the plant effluent.
When ferrous sulphate is used as a coagulant, the pH should be maintained above 9.5 to ensure complete precipitation of the iron. This is done by the addition of hydrated lime. The treated water should be corrected with the addition of carbon dioxide.
- Salt
Salts are compounds which contain both a cation and an anion. In water, the cation and the anion come apart and can interact with other charged particles in the water. All natural waters contain some concentration of cations and anions, such as calcium, sodium, magnesium, iron, manganese, sulphate, chloride, phosphate, and others. Some of these ions may affect the efficiency of the coagulation process.
- Alkalinity of water
The alkalinity of water is related to both the pH and the salts in the water. Alkalinity is the capacity of the water to neutralize acids, based on the water’s content of carbonate, bicarbonate, hydroxide, borate, silicate, and phosphate. Water with a high alkalinity is preferred for coagulation since it tends to have more positively charged ions to interact with the negatively charged colloids. To provide artificial alkalinity to water so as to have effective coagulation quick lime or hydrated lime are added to water.
- Quick lime
Quicklime or calcium oxide (CaO) may be used to provide artificial alkalinity to water when necessary. Quicklime varies in quality from 75 to 99% calcium oxide (typically 85%). The slaking of quick lime should be done carefully, ‘as the success of the water treatment depends to great extend on this process. The slaking requires 15 – 30 minutes under optimum conditions. The slaked lime is diluted with water and stored in solution tanks. As the calcium hydroxide formed by the slaking process is only slightly soluble, the solution in reality a suspension of the chemical. It is therefore necessary to agitate the contents of the tank continuously to maintain a uniform suspension.
The diluting water should be cold, because calcium hydroxide is more soluble in cold water than in warm water. Quicklime is used in water softening plants and at large water treatment plants because of its lower cost.
- Hydrated lime
Hydrated lime, also known as calcium hydroxide, is a white powder formed when quicklime is slaked in water. It does not deteriorate when stored, does not have to be slaked, and contains fewer impurities than most quicklime. The materials can be mixed directly in solution tanks and fed in dry form. Hydrated lime varies in quality between about 80% and 99% (typical 95%). Because hydrated lime is easily handled, its use is preferable in the smaller water treatment purification plants where lime is required to supply additional alkalinity to the water.
The alkalinity ratio of pure calcium oxide (CaO) to pure calcium hydroxide [Ca(OH)2] is 1 : 1.32.
44 Responses to “Water Treatment Coagulation”






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE
good notes on coagulation. send me more important notes on water and waste water
Good information but I want Comparison between FeCl3 & Non Ferric Alum
thank,for this information..
really this topic helps me to undestaning pre water treatement plant of thermal power plant
where i work
Thank You for giving this information
The information about ferric chloride got missed after ferric sulfate . plz include it
Thanks for your kind reminder. We will give the information of Ferric chloride on later post.
i require inf about use of iron salt as coagulant
You can get information about How Iron works as Coagulant.
http://www.thewatertreatments.com/waste-water-treatment-filtration-purify-sepration-sewage/coagulation-types
yes , this important , notices about Alum , so which is better Alum or Polymer ?
Alum is cheaper and better to use
good information abt coagulation and alum dosage . please send me all the details of water treatment process on my id…….
May i know that got any advantages dosing the flocculation in powder form for metal WWTP?
Advantage of Flocculation in powder form
No need dosage equipment
Reduction of the treatment costs
Reduction of Tensides content
Possibility of reuse of the water
Formation of easy to remove sludge
Complete clarification of the waste water
Reduction of COD.
By Admin
Thanks..this help me a lot for doing my waste water treatment thesis..
May i know that is activated sludge can substitute alum or nt ?
useful and valuable information for applictaion in my work in a water utility.please send me more details on treatment optimisation, especially of high DOC and color, High algal bloom, low turbididty raw waters
plz mail me about coagulants n anti coagulants
Very informative and useful, Thank you
-I’d like to know what happens to the sludge during this whole process? (does it become landfill? is it re used?) Could you please e-mail me, thank you.
If my company doing the waste water treatment plant for the mining slurry treatment, how much should i use for the 150m3/hr flow & 300m3/hr.
Very informative site about the topic. How does temperature effects coagulation? Some illustration required, better if on my e-mail. Thanks
THANKS FOR THE INFORMATION. I WILL BE MUCH HAPPY IF YOU KINDLY MAIL ME ALL THE WATER TREATMENT PROCESS.THANK YOU. STAY BLESS.
thanx for such information…really helpful… but i want the exact figured calculation about dosing of coagulant in pre-treatment of water in clark’s process of water softening…..
our normal flow is about 900-1200 cubic meter/hr..
and lime dosing calculation…?
this information is very useful.
Hi.
Some interesting graphics that help develop an understanding of how the treatment actually works.
I would like to hear your theories on ferric chloride as a coag on very high alkalinty drinking water i.e. 350 ppm or mg/litre. I require a final water UV @254nm transmitance of greater than 85%.
Regards
Vincent
I should say that i have already tried reducing the pH with sulfuric acid to pH 5.00-5.20.
in jar tests carried out the as the dose is increased the turbidity increases.
Thank you for this information! I propose the use of natural coagulant flocculation such as chitosan which are environmental friendly, not toxic like these chemicals.
It would be good to specify the trend of decreasing or increasing of alkalinity, pH and TDS in function of addition of coagulant.
Thank you ever so much!
Thank you for the info. I would like to ask about the ratio of alum and lime that i would need if i am going to purify water with a volume of 500ml. Please include also the procedures. I need it in my school experiment. Have a great day always!
thanks for that information but I wonder how practically determining the strength of alum or in other wards how to measure the Al2O3 content in alum
Thank you to share such information, but anybody let me know how to treat waste water which has 4.2 pH.
Which type of chemical i used to neutralize the water, kindly also describe the chemical with process.
Regard
These are wonderful information on the subject of coagulation and flocculation. I desire to know more on the type of flocculants that can be used for sewage water (suck-away). Pls I want urgent response.
so informative in waste water treatment and its components..
Hello, congratulations for the site. I have a question: my system of treatment using ferric chloride, lime and polyelectrolyte. The pH of the leachate that we treat is between 6.5 and 8. The ferric chloride may be replaced with another reagent most effective and economical? the real goal would be to reduce chloride discharge … Thanks!
Very informative site about the water treatment topic. How does Silicate effects in coagulation? Some illustration required, better if on my e-mail. Thanks
really helpful for science fair project.
a little details on reactions between the substances would help a lot…..
How to remove sodium silicate from waste water ?
do you have information about natural coagulants, especially about cactus.
Excellent text , thanks for water treatment coagulation
Ac0ually i thought this website given elaborate information for water treatment coagulation and sewage water treatments.Thanks for that info.
I scrolled u r site and got point for my feature idea in water treatment. i can get all idea in this site in sewage treatment coagulation and can attend interview for my jobs.
WE ARE SATISFIED YOUR SIDE.BUT GIVE ME CHEMICALLY REACTION.
thanks you..
it is very useful materiel for water and waster water treatment in power plants
would like to know more information on formation of coagulant especially to reduce organic, nickel and zinc contamination.
very good knowledge for me