Determination of Sulphate
Sulphate ions in water present usually in the form of compounds like CaSO4,MgSO4,Na2SO4 etc.
The determination of sulphate ions is water in based on the fact that when water is treated in cold with benzidine hydrochloride solution, the sulphate ions get precipitated as insoluble benzidine sulphate
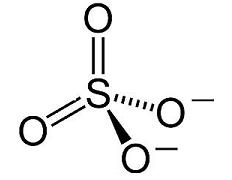
C12H8(NH2)2.2HCl + Na2SO4 ——-> C12H8(NH2)2.H2SO4 + 2NaCl
The precipitated is, however, Soluble in hot water and hydrolyses into benzidine and sulphuric acid
C12H8(NH2)2.H2SO4 + 2H2O —–> C12H8(HH2)2.2H2O + H2SO4
The free acid H2SO4 set free can be titrated against a standard caustic soda solution using phenolphthalein as indicator.
Procedure for Sulphate Analysis: –
Take 100 ml of water sample in a clean beaker. Add to it 40-50 ml of benzidine hydrochloric solution ( prepared hy disolving 4g of benzidine base in 10 ml of con.HCl ; and then diluting the solution to 1 litre with distilled water). Mix throughly giving a whirling motion to the beaker. Allow the beaker stand undistrubed for about 15 minutes, when all the sulphate ions will be precipitated as benzidine sulphate. Filter the precipate through a Whatman’s filter paper No.4. Wash the precipated on filter paper free of acid with minimum quantity of cold distilled water. Tranfer the filter paper along with precipate to a conical flask and add to it about 50 ml of distilled water and warm to about 50 degree C to dissolve the precipate. Then, titrate the liberated H2SO4 against N/50 caustic soda solution using phenolphatalein as indicator.
View Comments (7)
im a lab chemist, in my lab i'm determing the sulfate by below procedure.
300ml of distilled water.
25ml of sample water.
10ml of conc.HCl.
>>heating the beaker<>now 10ml of 5% of barium chloride is addinf<>agine heat for 5 mins<<
after this the beaker will kept on water bath for 2 hour.
calculation:
fianl-initial*1000*411.5/sample taken
i want to know is this correct or not coorect
Thanks&Regards
Abdul Gaffar - Senior Chemist.
What do mean by final and initial can you explain plz
i didnt get that how filter paper is used, whether it is washed or dipped in water into conical flask.
And, video makes better understand, please try to do that
Final and initial are ml quantity or weights please explain
Can any one tell me short method for determination of sulphate in water by titration or other method?
Short method of sulphate test?
fianl-initial*1000*411.5/sample taken please explain it with details.