

Water Quality and Standards
WATER QUALITY AND STANDARDS
- History of Water Quality Standards
- Importance of Water Quality Standards
- National and International Water Quality Standards
- Chemical and Bacteriological Characteristics
Water is very important for our life. Therefore, water must be free from organisms that are capable of causing disease and from minerals and organic substances that could produce adverse physiological effects (Pontius, 1990).
Drinking water should be aesthetically acceptable; it should be free from apparent turbidity, color, and odor and from any objectionable taste. Water meeting these conditions is termed ”Potable Water”. This means that it may be consumed in any desired amount without concern for adverse effects on health ( Pontius, 1990).
History of Water Quality Standards
Water quality was hot very well documented and people knew relatively little about disease as it related to water quality. Early historical treatment was performed only for the improvement of the appearance or taste of the water. No definite standards of quality other than general clarity or palatability were recorded by ancient civilizations. The first drinking water standards were issued at least 4000 years ago.
Hippocrates, the father of medicine, stated that ”water contributes much to health.” His interest in water centered on the purifying the most health- giving source of supply rather than on purifying the waters that were bad. Apparently, ancient people deduced by observation that certain waters promoted good health. While others produced infection.
By the 18th century, filtration of particles from water was established as an effective means of clarifying water. The general practice of making water clean was well recognized by that time, but the degree of clarity was not measurable.
The first municipal water filtration plant started operations in 1832 in Scotland. Aside from the frequent references of concern for the aesthetic properties of water, historical records indicate that standards for water quality were notable absent up to and including much of the 19th century.
With the realization that various epidemics (e.g., cholera and typhoid) had been caused and spread by water contamination, people saw that the quality of drinking water could not be accurately judged by sensory perception. Reliance on taste and smell was not an accurate means of judging the acceptability of water; more stringent quality criteria would be a necessary historical development. As a result, in 1852 a law was passed in London stating that all waters should be filtered.
In the mid-1890s, the Louisville Water Company, Louisville, combined coagulation with rapid sand filtration, significantly reducing turbidity and bacteria in the water.
The next major milestone in drinking water technology was the use of chlorine as disinfection. Chlorination was first used in 1908 and was introduced in a large number of water systems.
In the 19th century, the water quality standards was developed and regulated to give best potable water. These standards (Pontius, 1990) included the following :
- The basic formal and comprehensive review of drinking water concerns was launched.
- The concept of maximum permissible and safe limit was introduced.
- Physical and Chemical constituents were limited.
- Physical, Chemical, and bacteriological examinations were illustrated.
- Samples for bacteriological examination were to be obtained from pointes in the distribution system.
- Maximum concentrations, not to be exceeded always, where more suitable.
Importance of Water Quality Standards
Water quality standards normally identify the concentration of component properties shown by examinations of water samples to be safe, acceptable and attainable from available sources.
The maximum permitted concentration of various substances in public water supply is controlled throughout the world by legislation and varies to some extent from country to country.
Standards of water quality (Babbitt et al, 1962) can be divided into three types:
- For water of exceptionally great natural purity.
- For pure waters from a restricted area, and.
- For limits of matters permitted in water.
-
National and International Water Quality Standards
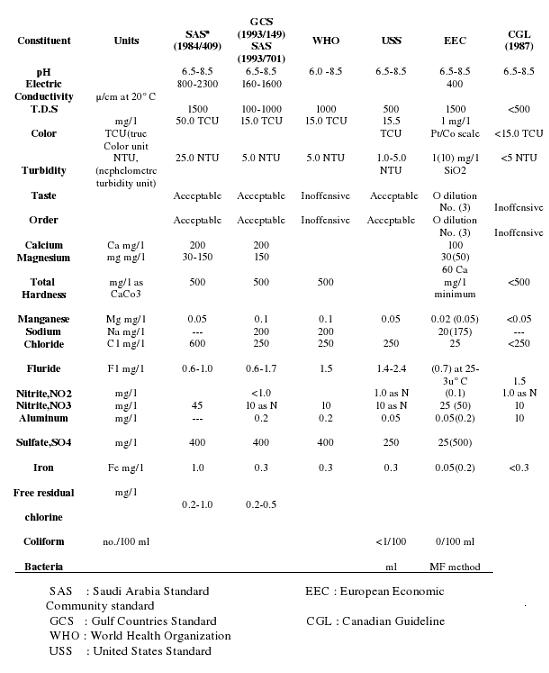
Several water quality standards were established and implemented. Some of these are still in use, whereas, some were modified. National (Saudi Arabian Standards) and selected international water quality standards for drinking water which are currently in use are presented in Table 3.1. Brief
information about these standards is presented below.
-
Saudi Arabian Standards SASO
Drinking water quality standards in Saudi Arabia (SAS) are issued by the Saudi Arabian Standards Organization (SASO). There are two drinking water quality standards currently in use in Saudi Arabia. These are the bottled drinking water quality standards (SASO, 1997) and the unbolted drinking water quality standards (GCS. 1993). The former have been issued in 1392 H (1972 G) by the Saudi Arabian Standards Organization (SASO) and implemented in 1405 H (1985 G). The un bottled drinking water quality standards have been issued in 1403 H (1982 G) by the Saudi Arabian Standards Organization (SASO and standardization and Metrology Organization for Gulf Council Countries (GSMO) and implemented in 1413 H (1993 G). The bottled drinking water quality standards are currently in use of the water quality in the distribution system.
-
Gulf Cooperation Countries Standards
Gulf Cooperation Council Countries Standards are the ”Unbottled Drinking Water Quality Standards” (GCS, 1993) which have been issued in 1403 H (1982 G) by the Saudi Arabia Standards Organization (SASO) and Standardization and Metrology Organization for Gulf Cooperation Council Countries (GSMO).
-
World Health Organization Guide for Drinking Water Quality
The primary aim of the World Health Organization (WHO) Guidelines for drinking water quality is the protection of public health and thus the elimination, or reduction to a minimum, of constituents in water that known to be hazardous to the health and well-being of the community
(Pontius, 1990).
-
USA Environmental Protection Agency for Drinking Water Regulations
United StatesEnvironmental Protection Agency (USEPA’s) secondary regulations set desirable levels for drinking water contaminants that may adversely affect the aesthetic value of drinking water. States may establish higher or lower levels that may be appropriate depending upon local conditions such as unavailability of alternative source water or other compelling factors, provided that public health and welfare are not adversely affected.
The present standards include maximum contaminant le4vel (MCL), also known as primary standards, for those organic and inorganic chemicals known to have toxic or carcinogenic effects, for turbidity, and for bacterial population. In addition, recommended contaminant levels. (RCL, secondary standards) have been established for certain contaminants, which are primarily of esthetic importance (Pontius, 1990).
-
European Economic Community Drinking Water Directives
The European Economic Community (EEC), having been established by a treaty of the Council of the European Communities, issued a council directive relating to the quality of water intended for human consumption. Specifically, the EEC standards provide for both the setting of standards to apply to toxic chemicals and bacteria that present a health hazard, and the definition of physical, chemical, and biological parameters for different uses of water. Specifically for the use of human consumption
(Pontius, 1990).
-
Canadian Drinking Water Guidelines
In Canadian, drinking water is a shared federal-provincial responsibility. In general, provincial governments are responsible for an adequate, safe supply, whereas the Federal Department of National Health and Welfare develops quality guidelines and conducts research. Guidelines for Canadian Drinking water quality (CGL) are developed through a joint federal-provincial mechanism and are not legally enforceable unlesss promulgated as regulations by the appropriate provincial agency. The first comprehensive Canadian drinking water guidelines were published by the Department of National Health and Welfare in 1968. They were
completely revised in 1978 and again in 1987. (Pontius, 1990).
Water Quality standard
Chemical Characteristics
-
pH
pH is a measured of the acidic or basic (alkaline) nature of a solution. The concentration of the hydrogen ion (H+) morality in a solution determines the pH.
Pure water has pH equal 7 and is neutral. Water with a pH less than 7 is acidic, and water with pH greater than 7 is basic. The principle system regulating pH in natural water is the carbonate system composed of carbon dioxide, carbonic acid, bicarbonate ions, and carbonate ions. PH is an important factor in the chemical and biological system of natural water. The degree of dissociation of weak acids or bases is affected by changes in pH.
-
Electrical Conductivity
Electrical conductivity is a numerical expression that shown the ability of water to hold electrical current. The unit of Electrical conductivity is mhos / cm. Electrical conductivity depends on the ionic forces of the solution, appearance of the dissolved ions and their concentration, relative concentration, and the measurement temperature.
-
Total dissolved Solid
Total dissolved Solid is the summation of all dissolves solids in the water, such as non-organic materials, carbonate, bicarbonate, nitrate, sodium, potassium, chloride, and magnesium. TDS affects the other characteristics
of drinking water such as taste and hardness.
-
Dissolved Oxygen
Dissolved Oxygen analysis measure the amount of gaseous oxygen dissolved an aqueous solution. Oxygen gets into water by diffusion from the surrounding air, by aeration, and as a waste product of photosynthesis. It generally ahs also been considered significant in the protection of aesthetic qualities.
Dissolved Oxygen concentration are an important tool to determine the ability of a water body to support a well-balanced aquatic fauna. Insufficient.
Dissolved Oxygen in the water column causes the anaerobic decomposition of organic materials leading to the formation of noxious gases, such has hydrogen sulfide, and the development of carbon dioxide an methane in sediments that bubble to the surface or which tend to float sludge.
Dissolved Oxygen in municipal water supplies is desired as an indicator of satisfactory water quality in terms of low residual of biologically available organic material. In addition, oxygen in water prevents the chemical reduction and subsequent leaching of iron and manganese, principally from the sediments. On the other hand, excess oxygen increases the rate of metal corrosion, which can increase the concentration of iron and other metals in drinking water supplies.
-
Alkalinity
The alkalinity of the water is its ability to neutralize an acid. It is the sum total of components in the water that tend to elevate the pH of the water
above 4.5. Carbonates, bicarbonates, phosphates, and hydroxide
contribute the common materials in natural water that increase alkalinity. Alkalinity resulting from naturally occurring materials is not considered a health hazard in drinking water supplies. Maximum levels up to 400 mg/L as calcium carbonates are not considered a problem.
-
Nitrates
The continuous interchange between atmospheric terrestrial nitrogen is referred to as nitrogen cycle. It has undergone profound modifications as a result of agricultural and industrial activities of man.
Atmospheric nitrogen is transformed by microbial action in plants and in the soil, by various atmospheric processes, and by industrial process compounds, such as ammonia, nitrates, and nitrites.
Nitrates are salts of nitric acid, most of which are readily soluble in water. Levels in cultivated soils, and thus levels in groundwater, may be increased by the use of commercial nitrogenous fertilizers and farm animal waste. Intensive animal farming produces large amounts of nitrogenous materials that may be converted into nitrates.
-
Chloride
Chloride ion is one of the major inorganic anions in water and wastewater. Chloride is a salt compound resulting from the combination of the gas, chlorine, and metal. The typical taste may be absent in waters containing as much as 1,000 mg/L when the predominant cautions are calcium and magnesium.
The chloride concentration is higher in wastewater than in raw water because sodium chloride is a common component of the diet and passes
unchanged through the digestive system. High chloride content may
metallic pipes and structures, as well as growing plants. Chloride can corrode metals and affect the taste of food products. Therefore, water is used in industry or processed for any use has a recommended maximum chloride level.
-
Calcium
The pressure of calcium in the form Ca2+ in water supplies as a result of passage through or over deposits of limestone, dolomite, gypsum, and gypsiferous shale. The calcium content may range from zero to several hundred milligrams per liter, depending on the source and treatment of the water. Calcium contributes to the total hardness of water.
-
Magnesium
Magnesium, in the form of Mg2+, ranks eighth among the elements in order of abundance and is a common constituent of natural water. Important contributes to the hardness of water, magnesium salts breaks down when heated, forming scale in boilers. The magnesium may very from zero to several hundred milligrams per liter, depending on the source and treatment of the water.
-
Sodium
Sodium, in the form of Na+, ranks sixth among the elements in order of abundance and is present in most natural waters. The levels may very from less than 1 mg Na/1, to more than 500 mg Na/1. The ratio of sodium to total cations is important in agricultural and human pathology.
-
Potassium
Potassium, in the form of K, ranks seventh among the elements in order of abundance, yet its concentration in most drinking waters seldom reaches 20 mg/L. However, occasional brines may contain more than 100 mg/L potassium.
-
Phosphorus
Phosphorus, in the form of P+, is particularly toxic and is subject to bioaccumulation in much same way as mercury. However, phosphorus as phosphate (PO4+) is one of the major mutrients required for plant nutrition and essential for life. Phosphorus enters waterways from several different sources.
The human body excretes about 1 pound per year of phosphorus. The use of phosphate detergents and other domestic phosphates increases the phosphorus load to natural habitats. Some industries, such as potato processing, have wastewater high in phosphates. It was found that total phosphorus concentrations in excess of 100 mg/L might interfere with coagulation in water treatment plants.
-
Ammonia
Ammonia in the form of NH3 is a pungent, colorless, gaseous, alkaline compound of nitrogen and hydrogen that is highly soluble in water. It is a biologically active compound present in natural waters as a normal biological degradation product of nitrogenous organic matter and wastewater. It may also reach surface waters through the discharge of industrial wastes containing ammonia as a byproduct or wastes from
industrial processes using ”ammonia water”.
-
Cadmium
Cadmium in the form of (Cd) occurs as a soft, blue-white, malleable metal or grayish-white powder. Cadmium has been shown to be toxic to man when ingested or inhaled. When ingested, it caused symptoms resembling food poisoning.
The naturally occurring presence of cadmium in the environment results mainly from gradual phenomena, such as rock erosion and abrasion, and of singular occurrences, such as volcanic eruptions. Cadmium is, therefore, naturally present in air, water, soil, and foodstuffs. It is soluble in acid, ammonium nitrate, and insoluble in water.
-
Lead
Lead in the form of (Pb) is a silver-gray soft metal that occurs in the earth’s at crust at an average concentration of about 13 mg/kg, however, some environment have much higher concentrations. Since these areas constitute a small percentage of total land, exposures of man to these sources are negligible.
People are exposed to lead by ingestion of food and fluids and by inhalation. Man’s intake of lead through water, particularly in urban areas, is generally low in comparison with exposure through air and food.
-
Chemical Oxygen Demand (COD)
Chemical oxygen demand is a measure of water pollution resulting from organic matter. STET the amount of oxygen required, or equivalent, for the oxidation of all chemically oxidyzable matter contained in a water
sample. This is accomplished using a number of methods utilizing a strong chemical oxidant. Boiling a solution containing chromic and sulfuric acids can digest the majority or organic matter.
Bacteriological Characteristics
-
Fecal coliform Bacteria
Total coliforms are measure of thee concentration of bacteria associated with the presence of sewage pollution. Fecal coliform bacteria are the most frequently applied microbiological indicators of water quality to determine the safety of water for drinking, swimming, and shellfish harvesting.
The coliform group is made up of a number of bacteria. Total coliform bacteria are all gram-negative asporogenous rods and have been associated with faces of warm-blooded animals and with soil. They are able to grow at 44.50C and ferment lactose, producing acid and gas. Use of fecal coliform bacteria has proven to be of more sanitary significance than the use of total coliform bacteria because to define water quality for swimming.
The presence of fecal coliform bacteria in aquatie environments indicates that the water has been contaminated with the fecal material of man or other animals. At the time this occurred, the source water might have been contaminated by pathogens or disease producing bacteria or viruses, which can also exist in fecal material. Some waterborne pathogenic diseases include typhoid fever, viral and bacterial gastroenteritis, and hepatitis A.
The presence of fecal contamination is an indicator that a potential health risk exists for individual exposed to this water, fecal coliform bacterial may occur in ambient water as a result of the overflow of domestic sewage or nonpoint sources of human and animal waste. Shellfish concentrates fecal coliform bacteria, other bacterial pathogens, and viruses found in water and sediment. Shellfish, as filter feeders, require a high quality of water in order to be microbiologically safe for human consumption, either raw or partially cooked.
One Response to “Water Quality and Standards”
Leave a Reply






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE
wish you all the best,
Regards.
A
AJITHKUMAR.