

Cholorination
Water Chlorination
Chlorination is the application of chlorine to the water for the purpose of disinfection. But the chlorination can also be used for taste and odor control, iron and manganese removal, and to remove some gases such as ammonia and hydrogen sulfide. Chlorination is currently the most frequently used form of disinfection in the water treatment field. However, other disinfection processes have been developed. Like several other water treatment processes, chlorination can be used as a pretreatment process (pre-chlorination) or as the final treatment of water (post -chlorination).
During pre-chlorination, chlorine is usually added to raw water after screening and before flash mixing. Post-chlorination, in contrast, is often the last stage in the treatment process. After flowing through the filter, water is chlorinated and stored in the clear water reservoir to allow a sufficient contact time for the chlorine to act. From the clear water reservoir, the water may be pumped into a service reservoir for storage and distribution to the consumers.
TYPES OF CHLORINATION
The different types of chlorination adopted in water treatment are as follows:
- Plain chlorination
- Pre-chlorination,
- Post-chlorination,
- Break point chlorination,
- Super chlorination,
- De-chlorination.
Plain chlorination:
When the raw water contains turbidity less than 10 NTU, obtained from unpolluted lakes or reservoirs, the water could be supplied to the public without any treatment except chlorination.
Such chlorination is called plain chlorination. The dosage of chlorine for plain chlorination is about 0.5 mg/L.
Pre-chlorination
Pre-chlorination is the addition of chlorine to the raw water prior to treatment to produce residual chlorine after meeting chlorine demand. The residual chlorine is useful in several stages of the reatment process – aiding in coagulation, controlling algae problems in sedimentation basins, reducing odor problems, and controlling mud-ball formation in filters. In addition, the chlorine has a much longer contact time when added at the beginning of the treatment process, so prechlorination increases safety in disinfecting heavily contaminated water.
Pre-chlorination is generally applied to the water before coagulation. It improves the coagulation and reduces load on filters. It also reduces taste, colour, odour, algae and other organisms. The chlorine dose for pre-chlorination should be 0.1 to 0.5 mg/L. The pre-chlorination is always followed by post chlorination, so as to ensure final safety of water. Until the middle of the 1970s, water treatment plants typically used both pre-chlorination and post-chlorination. However, the longer contact time provided by pre-chlorination allows the chlorine to react with the organics in the water and produce carcinogenic substances known as trihalomethanes (THM). As a result of concerns over THM, pre-chlorination has become much less common in the western countries. Currently, pre-chlorination is only used in plants where THM formation is not a problem.
Post chlorination
Post chlorination is the normal process of applying chlorine in the end, when all other treatments are completed but before the water reaches the distribution system. At this stage, chlorination is meant to kill pathogens and to provide a chlorine residual in the distribution system. Postchlorination is nearly always part of the treatment process, either used in combination with prechlorination or used as the sole disinfection process.
The chlorine dose at post-chlorination stage should be such as to leave a residual-chlorine of about 0.1 to 0.2 mg/L after a contact period of 20 to 30 minutes. This residual chlorine will ensure the disinfection of water if at all any recontamination occurs in the transmission and distribution system. Chlorine dose should not be generally greater than 2.0 mg/L as the excess residual concentration of chlorine may damage the pipelining and pump impellers.
Break point chlorination
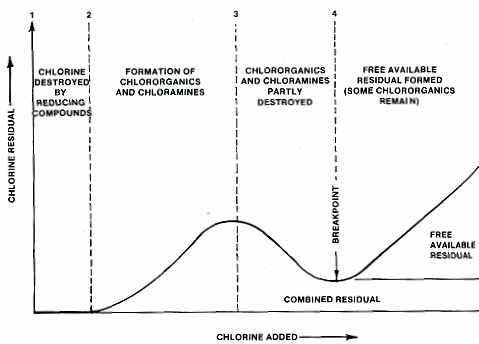
When chlorine is added to water, number of reactions taking place in water and the residual chlorine in water is also changing (increasingly as well as decreasingly). A typical breakpoint chlorination curve, showing the chemical reactions and the residual chlorine levels at various stages is illustrated in below figure.
Chlorine added to water first reacts with any iron, manganese or hydrogen sulphide that may be present in water. The entire chlorine added will be utilized in reacting with organic substances (including bacteria). Hence there will not be any residual chlorine (line AB) as initial chlorine demand. When chlorine is further added to water, it reacts with the ammonia present in the water, so as to produce combined chlorine residual (chloramines). The combi,ned chlorine residual, increases with addition of dosage (curve BC) until a maximum combined residual is reached (point C). If the addition of the chlorine is continued beyond the point C, the chlorine reacts with organics and ammonia naturally, found in the water and therefore, the residual chlorine content suddenly falls down, as shown in the curve CD. The point D at which the total chlorine demand is satisfied, as any chlorine added to water beyond this point, breaks through the water and appears as residual chlorine. This point D is called break point. The addition of chlorine beyond break point is called break point chlorination. The residual of free chlorine, appearing after break point, is not usually removed except by sun light and therefore, it takes care of the future recontamination of water. The breakpoint chlorination is the most common form of chlorination, in which enough chlorine is added to the water to bring it past the breakpoint and to create some free chlorine residual.
Super chlorination
Super chlorination is a term, which indicates the addition of excessive amount of chlorine (i.e., 5 to 15 mg/L) to the water. This may be required in some special cases when the water is highly polluted, or during epidemics of water borne diseases. The huge quantity of chlorine, which is added in super chlorination, is such as to give about 1 to 2 mg/L of residual beyond the break point in the treated water. Sometimes even higher doses may be used and the resultant -water is rechlorinated after the end of the desired contact period, by using dechlorinating agents.
De-chlorination
De-chlorination means removing the chlorine from the water. This is generally required when super-chlorination has been practiced. The de-chlorination process may either be carried out to such an extent that sufficient residual chlorine of 0.1 to 0.2 mg/L only remains in water after de-chlorination. The common de-chlorinating agents are sulphur dioxide gas, acivated carbon, sodium thiosulphiate, sodium metasulphate, and ammonia.
One Response to “Cholorination”
Leave a Reply






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE
What is over chlorination ?